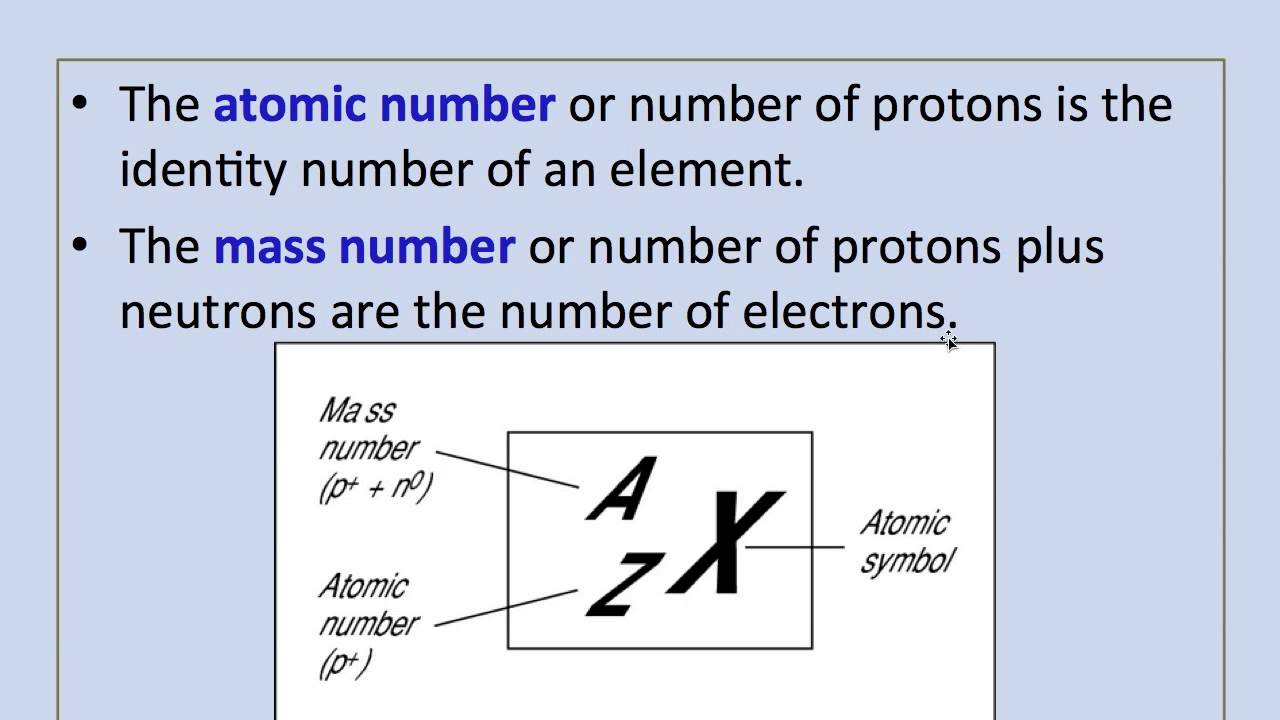
- The number of neutrons is found using mass number-atomic number. In the name aluminum-27, the 27 indicates the mass number. So the number of neutrons in aluminum-27 is 27-13 = 14.
- Here, atomic number of Aluminium is 13. It mans the number of electrons and protons are 13. Also, Mass number is the sum of number of protons and the number of neutrons. So, Number of neutrons = 27 - 13 = 14. Learn more, Atomic number and atomic mass.
Capitan mac os. ›› Aluminium Phosphate molecular weight. Molar mass of AlPO4 = 121.952899 g/mol. Convert grams Aluminium Phosphate to moles or moles Aluminium Phosphate to grams. Download whatsapp on chromebook. Molecular weight calculation: 26.981538 + 30.973761 + 15.9994.4 ›› Percent composition by element.
1 mole of Aluminium Oxide (Al2O3) = 2 × 27 + 3 × 16 = 102 g
We know, 102 g of Aluminium Oxide (Al2O3) = 6.022 × 1023 molecules of Aluminium Oxide (Al2O3)
We know, 102 g of Aluminium Oxide (Al2O3) = 6.022 × 1023 molecules of Aluminium Oxide (Al2O3)
We know, The number of atoms present in given mass = (Given mass ÷ molar mass) × Avogadro number
= (0.051 ÷102 ) × 6.022 × 1023 molecules of Aluminium Oxide (Al2O3)
= 3.011 × 1020 molecules of Aluminium Oxide (Al2O3)
The number of Aluminium ions (Al3+) present in one molecule of aluminium oxide is 2.
Therefore, the number of Aluminium ions (Al3+) present in 3.011 × 1020 molecules (0.051 g ) of Aluminium Oxide (Al2O3)
Mass Number Of Aluminium Ion
= 6.022 × 1020
This is an answered question from Chapter 3. Atoms And Molecules of CBSE Class 9th Science
What Is The Mass Number Of Aluminum
Check for Complete Exercise Solution for Chapter 3. Atoms And Molecules | CBSE Class 9th Science
