Pay Online Through the Account Center
Atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry. B: the atom considered as a source of vast potential constructive or destructive energy a largely forgotten legacy of this country's conquest of the atom. Broad when Congress passed the Atomic Energy Act in 1954 and allowed private utilities to 'harness the atom.' ATOM has a very specific total supply — 260,906,513 to be exact. Of these, at the time of writing, about 203,121,910 were in circulation. It is worth noting that these cryptocurrencies aren’t mined — instead, they are earned through staking. Two private sales were held in January 2017, followed by a.
Use our online Account Center to pay your bill, view past statements, view usage history, sign up for E-Bill manage your account information and more.
Log in to the Account Center to view and pay your bill from your computer, tablet or mobile device.
Automatic Monthly Payment
Stop worrying about late payments. Sign up for our Automatic Payment Plan to have your monthly payment automatically deducted from your financial institution or credit/debit card. We will still send you a monthly statement, but you won't have to worry about whether the bill gets paid on time.
Log in to the Account Center to sign up for Automatic Payment Plan.
If you don't want to log in to the Account Center, you can still make an online payment with the Make a One-Time Payment option. You will need your account number and your debit card, credit card, or checking account information.
Residential customers can pay by Visa, MasterCard, or Discover with no extra fee when using online payment methods or by calling our Customer Support Center at 888-286-6700. Atmos Energy does not accept credit card payments for commercial accounts.

If you are using Make a One-Time Payment, you can pay up to $350 more than your current balance. Enter the amount you want to pay in the Payment Amount field. If you choose to pay less than the full amount, please be aware that a late payment charge, if applicable, may be applied to any unpaid balance. Collection action may be taken unless you have made a payment arrangement in the Account Center or by calling a Customer Support Associate at 888-286-6700. If you are signed up for the Automatic Payment Plan, you will only be billed the Current Account balance each month.
Atom Software Download
Use a check or credit card to pay through our automated phone system by calling:
To pay by mail, please return the lower portion of your statement along with a check or money order in the envelope provided with your bill. The remittance address below should show through the envelope window. If you do not have a return envelope with your statement, your payment should be mailed to:
Atmos Energy
PO Box 740353
Cincinnati, OH 45274-0353
NOTE: Customers who mail their payment in an envelope that they address themselves, or those who use a corporate billing system or outside bill-paying service must use the address shown above.
Please do not send cash. To ensure your payment is credited properly, please write your account number on your check or money order.
Mailed payments take an average of 7 to 10 days to reach us and to be recorded in our system. If there's not adequate time for your payment to reach us by mail, please use one of the other payment options listed.

Pay with a debit card, credit card, personal check, bank draft, or money order when you visit an independently operated payment center. Locate a payment center in your area.
Atomic Blonde
Unlike planets orbiting the Sun, electrons cannot be at any arbitrary distance from the nucleus; they can exist only in certain specific locations called allowed orbits. This property, first explained by Danish physicist Niels Bohr in 1913, is another result of quantum mechanics—specifically, the requirement that the angular momentum of an electron in orbit, like everything else in the quantum world, come in discrete bundles called quanta.
Atomic Clock
In the Bohr atom electrons can be found only in allowed orbits, and these allowed orbits are at different energies. The orbits are analogous to a set of stairs in which the gravitational potential energy is different for each step and in which a ball can be found on any step but never in between.
The laws of quantum mechanics describe the process by which electrons can move from one allowed orbit, or energy level, to another. As with many processes in the quantum world, this process is impossible to visualize. An electron disappears from the orbit in which it is located and reappears in its new location without ever appearing any place in between. This process is called a quantum leap or quantum jump, and it has no analog in the macroscopic world.
Because different orbits have different energies, whenever a quantum leap occurs, the energy possessed by the electron will be different after the jump. For example, if an electron jumps from a higher to a lower energy level, the lost energy will have to go somewhere and in fact will be emitted by the atom in a bundle of electromagnetic radiation. This bundle is known as a photon, and this emission of photons with a change of energy levels is the process by which atoms emit light. See alsolaser.
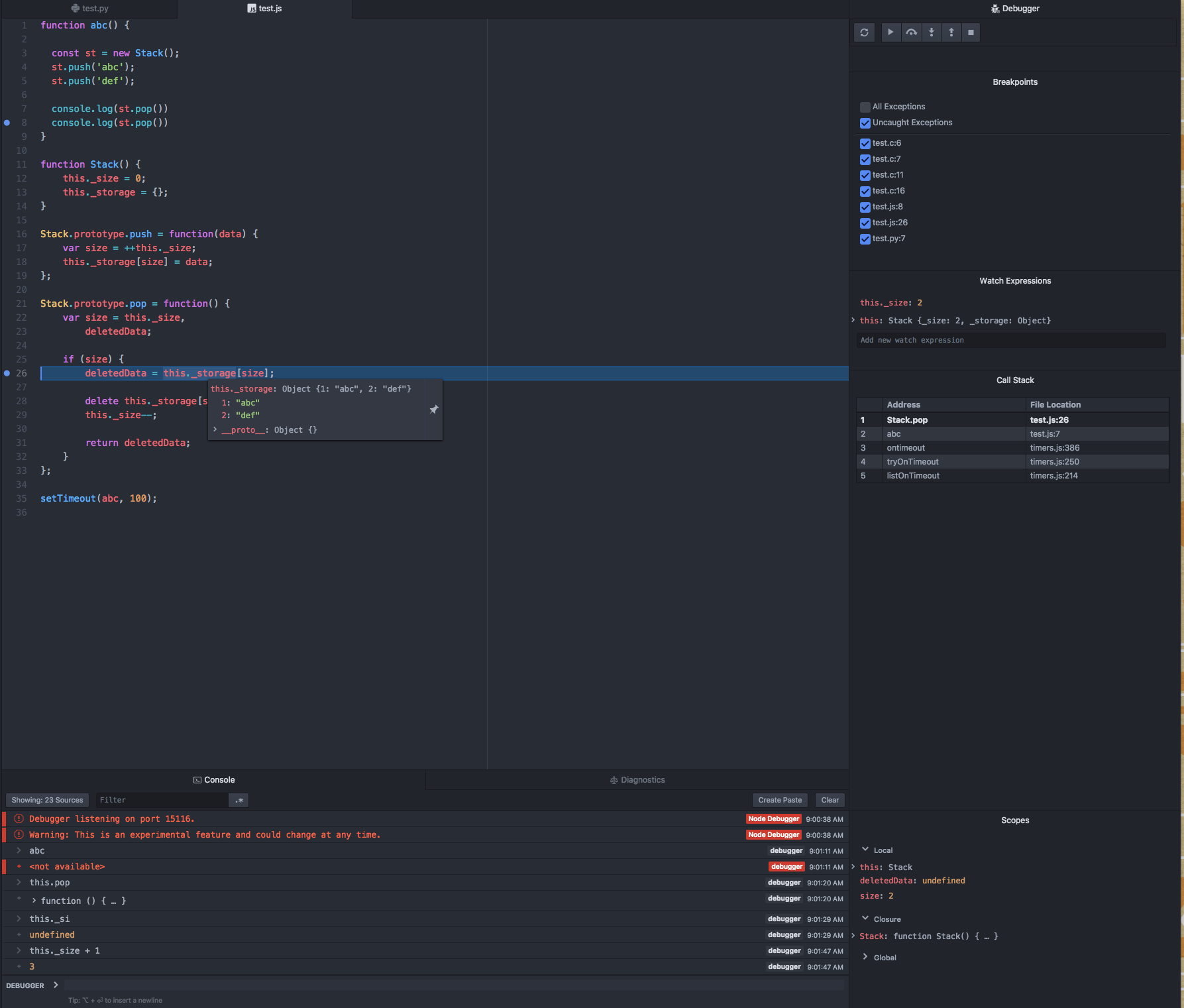
In the same way, if energy is added to an atom, an electron can use that energy to make a quantum leap from a lower to a higher orbit. This energy can be supplied in many ways. One common way is for the atom to absorb a photon of just the right frequency. For example, when white light is shone on an atom, it selectively absorbs those frequencies corresponding to the energy differences between allowed orbits.
Each element has a unique set of energy levels, and so the frequencies at which it absorbs and emits light act as a kind of fingerprint, identifying the particular element. This property of atoms has given rise to spectroscopy, a science devoted to identifying atoms and molecules by the kind of radiation they emit or absorb.
This picture of the atom, with electrons moving up and down between allowed orbits, accompanied by the absorption or emission of energy, contains the essential features of the Bohr atomic model, for which Bohr received the Nobel Prize for Physics in 1922. His basic model does not work well in explaining the details of the structure of atoms more complicated than hydrogen, however. This requires the introduction of quantum mechanics. In quantum mechanics each orbiting electron is represented by a mathematical expression known as a wave function—something like a vibrating guitar string laid out along the path of the electron’s orbit. These waveforms are called orbitals. See alsoquantum mechanics: Bohr’s theory of the atom.
